This monumental expansion underscores the increasing importance and complexity of medical device software. This guide aims to provide a comprehensive overview of the key considerations, processes, and best practices involved in navigating the dynamic and vital areas of healthcare.
Medical device software development encompasses the intricate process of designing, creating, validating, and maintaining software specifically intended to be used in conjunction with or as a medical device. This definition, however, opens up a spectrum of interpretations. It's crucial to differentiate between traditional medical devices with embedded software and the increasingly prominent category of Software as a Medical Device (SaMD).
The rise of digital health, personalized medicine, and telehealth has amplified the importance of robust and reliable medical device software. It enables advanced functionalities, enhances user experience, and improves healthcare outcomes by providing accurate data, facilitating remote monitoring, and automating complex procedures.
Developing medical device software is not merely about coding; it's about navigating a stringent regulatory environment. Agencies like the FDA in the US, the European Medicines Agency (EMA), national competent authorities in the EU, and other global bodies set forth rigorous standards to ensure the safety, effectiveness, and cybersecurity of these devices. Compliance with these regulations is paramount for market access and patient safety.
What is Medical Device Software Development?
At its core, medical device software development encompasses the intricate process of designing, creating, validating, and maintaining software specifically intended to be used in conjunction with or as a medical device. This definition, however, opens up a spectrum of interpretations.
It's crucial to differentiate between traditional medical devices with embedded software and the increasingly prominent category of Software as a Medical Device (SaMD).
Traditional medical devices, such as MRI machines, insulin pumps, or robotic surgery systems, often incorporate software as an integral component. In these cases, the software in medical devices is embedded, meaning it is part of the hardware and functions to control, monitor, or enhance the device's core functionalities. This embedded medical device software is crucial for the device's operation, but it's not considered a standalone medical device itself.
Software as a Medical Device (SaMD), on the other hand, is software that functions as a medical device on its own. It is not embedded in hardware but can be a standalone application, mobile app, web-based platform, or cloud service. SaMD performs medical functions, such as diagnosis, prevention, monitoring, treatment, or alleviation of disease, without being physically connected to a medical device in the traditional sense.
Understanding the nuances between these categories is critical for anyone involved in medical device software development. Both embedded software and SaMD are subject to rigorous regulatory scrutiny and require adherence to stringent medical device software standards. However, the development pathways, validation processes, and software risk management processes differ based on the type of software and its intended use.
Therefore, a clear grasp of what constitutes medical device software and the different categories within it is the foundational first step for successful development and market entry.
Classification Systems for Medical Device Software
Regulatory bodies worldwide classify medical devices, including software, based on their intended use and the potential risk they pose to patients. These classifications dictate the level of regulatory scrutiny and the requirements for development, testing, and documentation.
- FDA Classification (USA): The FDA classifies medical devices into three classes based on risk:
- Class I (Low Risk): General controls are sufficient to assure safety and effectiveness (e.g., general wellness apps, some dental instruments).
- Class II (Moderate Risk): General controls alone are insufficient, requiring special controls (e.g., infusion pumps, powered wheelchairs).
- Class III (High Risk): Requires premarket approval (PMA) due to high risk or life-sustaining/supporting nature (e.g., pacemakers, HIV diagnostic tests).
- EU MDR Classification (Europe): The EU Medical Device Regulation (MDR) classifies devices into four classes (I, IIa, IIb, III) based on factors like invasiveness, duration of contact, and whether they administer medicinal products. SaMD is often classified based on the "level of impact" on patient health, ranging from non-critical (Class I) to critical (Class III).
- IEC 62304 Software Safety Classification: IEC 62304, a key standard for medical device software lifecycle processes, classifies software based on the potential consequences of software failure:
- Class A (No injury or damage to health): Software failure is unlikely to cause injury.
- Class B (Non-serious injury): Software failure could cause non-serious injury.
- Class C (Serious injury or death): Software failure could cause serious injury or death.
The classification of medical device software significantly impacts the development process. Higher-risk classifications demand more rigorous documentation, extensive testing, more stringent risk management, and often require clinical data for regulatory approval. Developers must understand these classifications early in the development cycle to ensure compliance and streamline the regulatory pathway.
Key Challenges and Solutions:
- Complexity and Interoperability: Integrating diverse systems and ensuring seamless data flow can be challenging. Solution: Employ modular design, establish clear APIs, and adhere to industry standards for interoperability (e.g., FHIR, DICOM).
- Cybersecurity Threats: Medical devices are attractive targets for cyberattacks, risking patient data and device functionality. Solution: Implement a "security by design" approach, conduct rigorous threat modeling, and ensure continuous vulnerability management.
- Evolving Regulatory Landscape: Staying abreast of dynamic regulations is crucial. Solution: Maintain a dedicated regulatory affairs team, engage with regulatory bodies, and adopt agile compliance strategies.
- Testing and Validation: Ensuring software performs as intended in diverse clinical scenarios is complex. Solution: Adopt a risk-based testing approach, utilize simulated and real-world environments, and document all validation activities thoroughly.
Examples of Innovative Software as a Medical Device
Examples of software as a medical device are diverse and rapidly expanding, encompassing everything from AI-powered diagnostic tools analyzing medical images to mobile apps that monitor patient vital signs and deliver therapeutic interventions.
The landscape of Software as a Medical Device (SaMD) is rapidly evolving, showcasing remarkable innovation and transformative potential within healthcare. To truly appreciate the impact of medical device software development, it’s essential to explore concrete examples of SaMD that are revolutionizing patient care and healthcare delivery.
AI-powered diagnostic Imaging Software
One of the most prominent examples of SaMD lies in the realm of medical imaging. AI algorithms are being developed and deployed as standalone software as medical devices to analyze DICOM medical imaging software data, such as X-rays, CT scans, MRIs, and pathology slides. These sophisticated programs can detect subtle anomalies, identify patterns indicative of disease, and assist radiologists in making faster and more accurate diagnoses.
Examples include software that detects cancerous nodules in lung scans, algorithms that identify early signs of diabetic retinopathy in retinal images, and programs that analyze brain scans for indicators of Alzheimer’s disease. These AI-driven tools are significantly enhancing diagnostic accuracy and efficiency, leading to earlier interventions and improved patient outcomes.
Remote Patient Monitoring (RPM) Software
- Remote patient monitoring software represents another powerful application of SaMD. These platforms leverage wearable sensors, connected devices, and mobile apps to collect patient physiological data outside of traditional clinical settings. The remote patient monitoring software then analyzes this data, providing real-time insights to healthcare providers.
Examples include apps that monitor blood glucose levels for diabetic patients, platforms that track cardiac rhythms for individuals with heart conditions, and systems that monitor respiratory function for patients with chronic lung disease. RPM software empowers proactive care management, early detection of deteriorating conditions, and reduced hospital readmissions, fundamentally changing how chronic diseases are managed.
Mental Health and Behavioral Therapy Apps
The increasing recognition of mental health as a critical component of overall well-being has spurred the development of SaMD in the form of mental health and behavioral therapy apps. These software programs, such as medical device examples, offer a range of therapeutic interventions, including cognitive behavioral therapy (CBT), mindfulness exercises, and stress management techniques, which are delivered directly to patients via their smartphones or tablets.
These apps can provide accessible and affordable mental health support, particularly in underserved areas, offering tools for managing anxiety, depression, and other mental health conditions.
Personalized Medicine Software
The promise of personalized medicine is being realized through the development of SaMD, which analyzes patient-specific data, including genomic information, lifestyle factors, and medical history, to tailor treatment plans.
This sophisticated software, as a medical device program, can predict treatment response, optimize drug dosages, and guide clinical decision-making based on an individual’s unique biological profile. This approach leads to more targeted and effective therapies, minimizing adverse effects and maximizing positive outcomes.
These examples are just the tip of the iceberg. The field of SaMD is rapidly expanding, with innovations emerging in areas like surgical planning software, rehabilitation and physical therapy apps, and digital therapeutics that deliver evidence-based treatments directly to patients.
The continued advancement of software as a medical device is poised to revolutionize healthcare further, offering unprecedented opportunities to improve patient care, enhance diagnostic capabilities, and transform the delivery of medical services.
Types of Medical Device Software
The spectrum of medical device software is incredibly diverse, reflecting the varied needs and applications within the healthcare ecosystem. Categorizing these types helps to understand their functionalities, regulatory requirements, and development considerations.
We can broadly classify software in medical devices into several key categories:
Embedded Software
As mentioned earlier, embedded software is integral to traditional medical devices. It resides within the hardware of devices like pacemakers, infusion pumps, ventilators, and imaging equipment.
This type of medical device software directly controls and manages the device's functions, ensuring precise operation, data acquisition, and real-time responses. Stringent real-time performance requirements, high reliability, and robust safety features often characterize embedded software.
Standalone Software as a Medical Device (SaMD)
This category encompasses software that functions independently as a medical device, as discussed in detail in previous sections. SaMD can be deployed as mobile apps, web applications, desktop software, or cloud-based services.
Its versatility allows for a wide range of applications, from diagnostic tools and remote patient monitoring software to therapeutic interventions and personalized medicine platforms.
Infrastructure Software
This type of software and medical systems supports the broader healthcare IT infrastructure. It includes software that manages medical records (Electronic Health Records - EHRs), Picture Archiving and Communication Systems (PACS) for DICOM medical imaging software, Laboratory Information Management Systems (LIMS), and hospital information systems (HIS).
While not directly interacting with patients like SaMD, infrastructure software is crucial for efficient healthcare operations, data management, and interoperability.
Medical Device Data Systems (MDDS)
MDDS software is designed to transfer, store, convert formats, and display medical device data. It is often used in conjunction with other medical devices to facilitate data aggregation and analysis.
MDDS is not intended to actively control medical devices or directly alter their functions, but rather to manage and present the data they generate for clinical review and decision-making.
Software for Manufacturing and Quality Control
This category of software and medical device manufacturing process is critical for ensuring the quality and compliance of medical devices. It includes software used in the design, development, manufacturing, testing, and quality assurance stages of medical device production.
These systems help streamline processes, track data, ensure adherence to medical device software standards, and maintain comprehensive audit trails, which are crucial for regulatory compliance.
Understanding these different types of medical device software is crucial for startup owners and CXOs venturing into this space. Each type presents unique development challenges, regulatory pathways, and market opportunities. Choosing the correct kind of software to focus on, based on market needs and business capabilities, is a key strategic decision.
Benefits of Medical Device Software Development
Investing in medical device software development offers a plethora of benefits for healthcare providers, patients, and businesses alike. The advantages extend beyond improved clinical outcomes and encompass enhanced efficiency, cost-effectiveness, and new revenue streams.
For startup owners and CXOs, understanding these benefits is crucial for making informed investment decisions and capitalizing on the growing market.
Enhanced Diagnostic Accuracy and Efficiency
AI-powered medical devices software, particularly in imaging and diagnostics, significantly improves the accuracy and speed of disease detection. Algorithms can identify subtle anomalies that may be missed by the human eye, leading to earlier and more precise diagnoses.
This translates to faster treatment initiation, improved patient outcomes, and reduced healthcare costs associated with delayed diagnoses.
Improved Patient Outcomes and Personalized Care
Software as a medical device enables personalized and proactive healthcare management. Remote patient monitoring software empowers continuous data collection and analysis, allowing for early detection of deteriorating conditions and timely interventions.
Personalized medicine software tailors' treatment plans based on individual patient profiles, leading to more effective therapies and reduced adverse effects. These advancements contribute to significantly improved patient outcomes and a more patient-centric approach to healthcare.
Increased Efficiency and Reduced Healthcare Costs
Medical device software can automate many time-consuming and manual tasks in healthcare workflows. AI-powered diagnostic tools can streamline image analysis, RPM software can reduce the need for frequent in-person clinic visits, and digital therapeutics can deliver scalable and cost-effective interventions.
These efficiencies translate to reduced healthcare costs for both providers and patients while simultaneously freeing up healthcare professionals to focus on more complex patient needs.
Expanded Access to Healthcare, Especially in Remote Areas
Remote patient monitoring software and mobile health apps extend healthcare access to underserved populations, particularly in rural or remote areas where access to specialists and healthcare facilities is limited.
Software as a medical device can deliver diagnostic services, monitoring, and therapeutic interventions remotely, breaking down geographical barriers and improving healthcare equity.
New Revenue Streams and Business Opportunities
The rapidly growing market for medical device software development presents significant business opportunities for startups and established companies alike.
Developing innovative SaMD solutions, offering healthcare software development services in the medical device space, can unlock substantial revenue streams and establish market leadership in a high-growth sector.
Enhanced Data-Driven Decision Making
Medical device software generates vast amounts of valuable data. This data, when properly analyzed, provides insights that can drive evidence-based decision-making at all levels of healthcare.
From optimizing clinical workflows to identifying population health trends and informing public health policies, data derived from medical device software is a powerful asset for improving the healthcare system as a whole.
In essence, the benefits of medical device software development are multifaceted and far-reaching. By embracing innovation in this space, healthcare stakeholders can drive improvements in patient care, enhance operational efficiency, and unlock significant economic value, contributing to a more sustainable and effective healthcare system.
Step-by-Step Process to Build Custom Medical Device Software
Developing custom medical device software is a complex undertaking that demands meticulous planning, rigorous execution, and unwavering adherence to regulatory standards. A structured, step-by-step process is crucial for navigating this intricate landscape and ensuring the successful creation of safe, effective, and compliant software.
Here's a breakdown of the key stages involved in building software development for medical devices:
1. Planning and Requirements Gathering
The initial phase is critical for defining the scope, objectives, and requirements of the medical device software. This involves:
- Defining the Intended Use: Clearly articulate the medical purpose of the software, the target patient population, and the clinical problem it aims to solve.
- Identifying User Needs: Gather input from clinicians, patients, and other stakeholders to understand their needs and expectations from the software.
- Defining Functional and Non-Functional Requirements: Specify what the software must do (functional requirements) and how it must perform (non-functional requirements, e.g., performance, security, usability).
- Risk Assessment (Preliminary): Conduct an initial risk assessment to identify potential hazards associated with the software and categorize the risk level, which will inform the development process intensity.
Pro Tips:- For Compliance with IEC 62304 Planning: At this stage, specifically consider how the software safety classification (per IEC 62304) will influence the rigor of your development plan. This includes defining the software development lifecycle (SDLC) model (e.g., Waterfall, Agile, V-model, or a hybrid approach) and tailoring it to meet the requirements of your software safety class. Document your chosen SDLC and the activities for each phase.
2. Design and Architecture
Based on the defined requirements, the next step is to design the software architecture and detailed specifications:
- Software Architecture Design: Define the overall structure of the software, including modules, components, interfaces, and data flow. Consider scalability, security, and maintainability.
- Detailed Design Specifications: Develop detailed specifications for each software component, outlining algorithms, data structures, user interface design, and integration points.
- Cybersecurity Planning: Integrate medical device cybersecurity considerations into the design phase, planning for security controls and data protection measures.
Pro Tips: Integration of ISO 14971 Principles: Incorporate risk management activities as outlined in ISO 14971 (Risk Management for Medical Devices) directly into the design phase. This involves identifying potential software-related hazards (e.g., incorrect calculations, data corruption, timing issues), estimating and evaluating associated risks, and designing risk control measures (e.g., error handling, redundancy, warnings) to reduce risks to acceptable levels. Document all risk control measures and their verification methods.
3. Software Development and Coding
This is the core development phase where the software is written:
- Coding and Implementation: Write the software code based on the detailed design specifications, adhering to coding best practices and medical device software standards.
- Version Control and Configuration Management: Implement robust version control systems to track changes, manage different versions, and facilitate collaboration.
- Unit Testing: Conduct thorough unit testing of individual software modules to ensure they function correctly and meet specified requirements.
Pro Tips: Adherence to IEC 62304 Development Processes: Ensure that coding practices, code reviews, and module testing align with the rigor defined by your IEC 62304 software safety class. This may involve specific coding standards, static code analysis, and thorough peer reviews for higher safety classes.
4. Testing and Verification
Rigorous testing is paramount for medical device software testing to ensure safety, effectiveness, and compliance:
- Integration Testing: Test the interactions between different software modules and with external systems to ensure seamless integration.
- System Testing: Test the entire software system as a whole, verifying that it meets all functional and non-functional requirements.
- Usability Testing: Evaluate the medical device's software testing usability with representative users to ensure it is intuitive and easy to use in a clinical setting.
- Security Testing: Conduct thorough security testing to identify and mitigate vulnerabilities, ensuring medical device cybersecurity.
- Risk-Based Testing: Prioritize testing efforts based on the software's safety classification and identified risks. Critical functions and components associated with high risks require more extensive and rigorous testing. This involves designing specific test cases for known hazards and implementing comprehensive regression testing.
Critically, validate any software tools used in the development process that could impact the medical device software's safety or performance (e.g., compilers, static analysis tools, test automation frameworks). This ensures their reliability and suitability for their intended purpose in a regulated environment.
5. Validation and Regulatory Compliance
This stage focuses on demonstrating that the software meets regulatory requirements and is fit for its intended use:
- Validation Testing: Conduct validation testing in a simulated or real-world clinical environment to confirm that the software performs as intended and achieves its medical purpose.
- Documentation and Traceability: Maintain comprehensive documentation throughout the development process, ensuring traceability between requirements, design, code, testing, and validation. This is crucial for demonstrating compliance with medical device software standards.
- Regulatory Submission: Prepare and submit the required regulatory documentation (e.g., to the FDA in the US or notified bodies in Europe) for market clearance or approval, depending on the risk classification of the software.
Ensure all documentation and validation activities align with key standards like ISO 13485 (Quality Management Systems for Medical Devices), IEC 62304 (Software Lifecycle Processes), and ISO 14971 (Risk Management for Medical Devices). For the FDA, this means adhering to 21 CFR Part 820 (Quality System Regulation) and relevant guidance documents (e.g., "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices"). For the EU, conformity with MDR is paramount.
Utilize structured documentation approaches such as Design History Files (DHF) to consolidate all design and development records, Device Master Records (DMR) for specifications and manufacturing, and Device History Records (DHR) for production and quality control. Employ electronic document management systems (EDMS) to ensure version control, audit trails, and accessibility.
6. Deployment and Post-Market Surveillance
Once regulatory clearance is obtained, the software can be deployed:
- Deployment and Installation: Plan and execute the deployment of the software to the intended users, ensuring proper installation and training.
- Post-Market Surveillance: Establish a system for ongoing monitoring of the software's performance in the field, collecting user feedback, and tracking any adverse events or issues. This is crucial for the software risk management process and continuous improvement.
- Maintenance and Updates: Plan for ongoing maintenance, bug fixes, security updates, and potential software enhancements based on user feedback and evolving needs.
- Software Change Control: Implement a robust change control process for all software updates, bug fixes, and new features. Any modification to the software, even minor ones, must be assessed for their impact on safety, performance, and regulatory compliance. This often requires re-verification, re-validation, and potentially new regulatory submissions, primarily if the change affects the intended use or safety of the device. Maintain detailed documentation for all changes, including problem resolution and risk management activities.
This step-by-step process provides a general framework. The specific details and intensity of each stage will vary depending on the complexity, risk classification, and intended use of the medical device's software. However, adhering to a structured approach and prioritizing quality, safety, and regulatory compliance is essential for success in this demanding but gratifying field.
Key Considerations When Developing Medical Device Software
Developing medical device software demands careful attention to critical factors that impact patient safety and regulatory compliance. For startup owners and CXOs, prioritizing these considerations is essential for building robust and successful solutions.
Regulatory Compliance
Meeting medical devices software standards (FDA, MDR/IVDR, ISO 13485) is paramount. Compliance isn't optional; it's integral throughout development for market access. Understand your regulatory pathway (510(k), De Novo, PMA).
Adherence to key international standards is critical. This includes:
- ISO 13485: Specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
- IEC 62304: Defines the software lifecycle processes required for medical device software. It categorizes software into safety classes (A, B, C) that dictate the rigor of development, testing, and documentation.
- ISO 14971: Provides a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (IVD) medical devices, to estimate and evaluate the associated risks, to control these risks, and to monitor the effectiveness of the controls.
- FDA Regulations (e.g., 21 CFR Part 820, relevant guidance documents): The FDA provides comprehensive guidance on software validation, cybersecurity, and SaMD development.
- EU MDR (Medical Device Regulation): The MDR significantly increased the regulatory requirements for medical devices, including software, in the European Union, emphasizing clinical evidence, post-market surveillance, and unique device identification (UDI).
Implement and maintain a robust QMS that is certified to ISO 13485. This QMS serves as the backbone for all development activities, ensuring consistency, traceability, and continuous improvement. It mandates rigorous documentation, risk management, and post-market surveillance procedures.
Stay abreast of evolving regulations concerning cybersecurity (e.g., FDA's recent guidance on "Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions") and the increasing scrutiny on AI/ML in medical devices (e.g., FDA's "Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan"). These areas require specific considerations for data integrity, algorithm bias, and continuous learning validation.
Safety and Risk Management
Patient safety is the top priority. Implement a robust software risk management process to identify, analyze, and mitigate potential hazards at each stage. Prioritize risk control for patient safety. Risk management must be an ongoing activity throughout the entire software lifecycle, as per ISO 14971. This involves:
- Hazard Identification: Systematically identify all potential software-related hazards (e.g., software defects, erroneous data, timing errors, unhandled exceptions, incorrect user interface elements). Use techniques like FMEA (Failure Mode and Effects Analysis) specific to software.
- Risk Estimation and Evaluation: Assess the probability of occurrence and the severity of harm for each identified hazard. Determine the acceptability of the risk according to a predefined risk acceptance criterion.
- Risk Control Measures: Design and implement specific risk control measures within the software (e.g., input validation, error logging, redundancy, fail-safe mechanisms, warnings to users, limiting ranges of values). These must be traceable to the identified hazards.
- Verification of Risk Control Effectiveness: Critically, verify that the implemented risk control measures are effective in reducing risks to an acceptable level. This often involves specific testing and validation activities.
- Residual Risk Evaluation: After all controls are implemented, evaluate the remaining (residual) risks to ensure they are acceptable.
- Software Safety Classification Impact: The software safety classification (IEC 62304 Class A, B, or C) directly influences the rigor of risk management activities. Class C software demands the most exhaustive hazard analysis, risk control implementation, and verification.
Cybersecurity
Medical device cybersecurity is crucial. Protect patient data and device functionality from cyber threats. Implement strong security controls, follow best practices, and conduct regular security testing.
- "Security by Design" Principles: Integrate security considerations from the earliest design phases, rather than as an afterthought. This includes defining security requirements alongside functional requirements.
- Threat Modeling: Conduct systematic threat modeling to identify potential vulnerabilities and attack vectors specific to your software and its operating environment. This helps in proactively designing security controls.
- Vulnerability Management: Establish a robust process for identifying, assessing, and remediating software vulnerabilities throughout the lifecycle. This includes using static and dynamic application security testing (SAST/DAST) tools and conducting penetration testing.
- Security Risk Assessment: Perform a comprehensive security risk assessment, distinct from traditional safety risk assessment, to evaluate the likelihood and impact of cybersecurity threats.
- Software Bill of Materials (SBOM): Generate and maintain an SBOM to identify all open-source and third-party components used in the software. This helps in tracking and addressing vulnerabilities in supply chain software.
- Ongoing Monitoring and Incident Response: Implement continuous monitoring for security threats post-market. Develop a detailed incident response plan to address and mitigate any discovered cybersecurity vulnerabilities or breaches quickly. Regulatory bodies like the FDA increasingly require post-market cybersecurity management plans, including patch management and coordinated vulnerability disclosure.
Data Privacy and Security
Comply with data privacy regulations (HIPAA, GDPR). Implement robust data security measures like encryption and access controls to protect sensitive patient information.
Usability and Human Factors
Design for ease of use in clinical settings. Apply human factors engineering, incorporate user feedback, and conduct usability testing to ensure intuitive and safe software for clinicians.
Interoperability and Integration
Ensure seamless software and medical system integration. Adhere to standards (HL7, DICOM) for interoperability with EHRs, PACS, and other systems for efficient data exchange.
Validation and Verification
Rigorous validation and verification are fundamental. Prove that the software works as intended and meets requirements. Comprehensive testing and traceable documentation are essential for compliance and confidence.
Employ a multi-layered testing approach, including:
- Unit Testing: Verify individual code modules.
- Integration Testing: Confirm interfaces and interactions between modules.
- System Testing: Validate the entire system against requirements.
- Usability Testing: Assess ease of use and human interaction in realistic scenarios.
- Security Testing: Identify vulnerabilities and weaknesses.
- Performance Testing: Evaluate speed, responsiveness, and stability under load.
- Regression Testing: Ensure new changes don't negatively impact existing functionality.
Software validation is crucial and must demonstrate that the software fulfills its intended use in a relevant clinical environment. This goes beyond verifying requirements and focuses on fitness for purpose in real-world settings.
Pro Tips: All testing and validation activities must be meticulously documented. This includes test plans, test cases, test results, defect tracking, and release notes. This documentation provides objective evidence for regulatory bodies that the software is safe and effective. The level of detail for documentation directly corresponds to the software's safety classification.
SDLC Management
Choose and adhere to a defined Software Development Lifecycle (SDLC) model (Agile, Waterfall, Hybrid). Document your SDLC and tailor it to project needs and risks.
By carefully considering these key factors throughout the medical device software development process, startup owners and CXOs can significantly increase their chances of building successful, compliant, and impactful medical device software solutions that truly benefit patients and the healthcare system.
Challenges in Medical Device Software Development
Developing medical device software is inherently challenging due to strict regulations, complex healthcare tech, and the critical focus on patient safety. Startup owners and CXOs should be aware of these common pitfalls for proactive planning.
Regulatory Complexity
Medical device software development companies navigate evolving medical device software standards and regulations (FDA, MDR/IVDR) across regions that are complex and resource-intensive. Continuous compliance demands expertise.
Cybersecurity Threats
Connected medical device software faces increasing cyber threats (ransomware, breaches). Robust medical device cybersecurity measures and vigilance are essential to protect patient data and device function.
Safety & Risk Management
Demonstrating safety and effectiveness is core. Developing an intense software risk management process, conducting thorough assessments, and implementing controls require specialized skills.
Talent Acquisition
Finding talent with specialized skills in software engineering, regulations, cybersecurity, and healthcare workflows for medical device software development is difficult, especially for startups.
Validation & Verification Complexity
Rigorous validation and verification to meet regulatory demands are complex and costly. Comprehensive testing, documentation, and traceability are crucial but demanding.
Interoperability Issues
Seamless integration with diverse healthcare IT systems (building EHRs or PACS) is challenging due to varying standards and protocols. Requires careful planning and custom solutions.
Legacy Systems & Tech Debt
Integrating with outdated healthcare IT infrastructure and managing technology debt adds complexity and challenges to modernization efforts.
Time-to-Market vs. Rigor
Balancing rapid innovation with the rigorous and time-consuming nature of compliant medical device software development creates tension. Finding the right balance is key.
Addressing these challenges requires a strategic approach, strong quality systems, skilled teams, and regulatory/technical expertise. Proactive planning and awareness are vital for startup owners and CXOs to succeed in this demanding field.
Best Practices for Superior Medical Device Software
To build exceptional medical device software and overcome development challenges, adopting these best practices is crucial for safety, compliance, and quality.
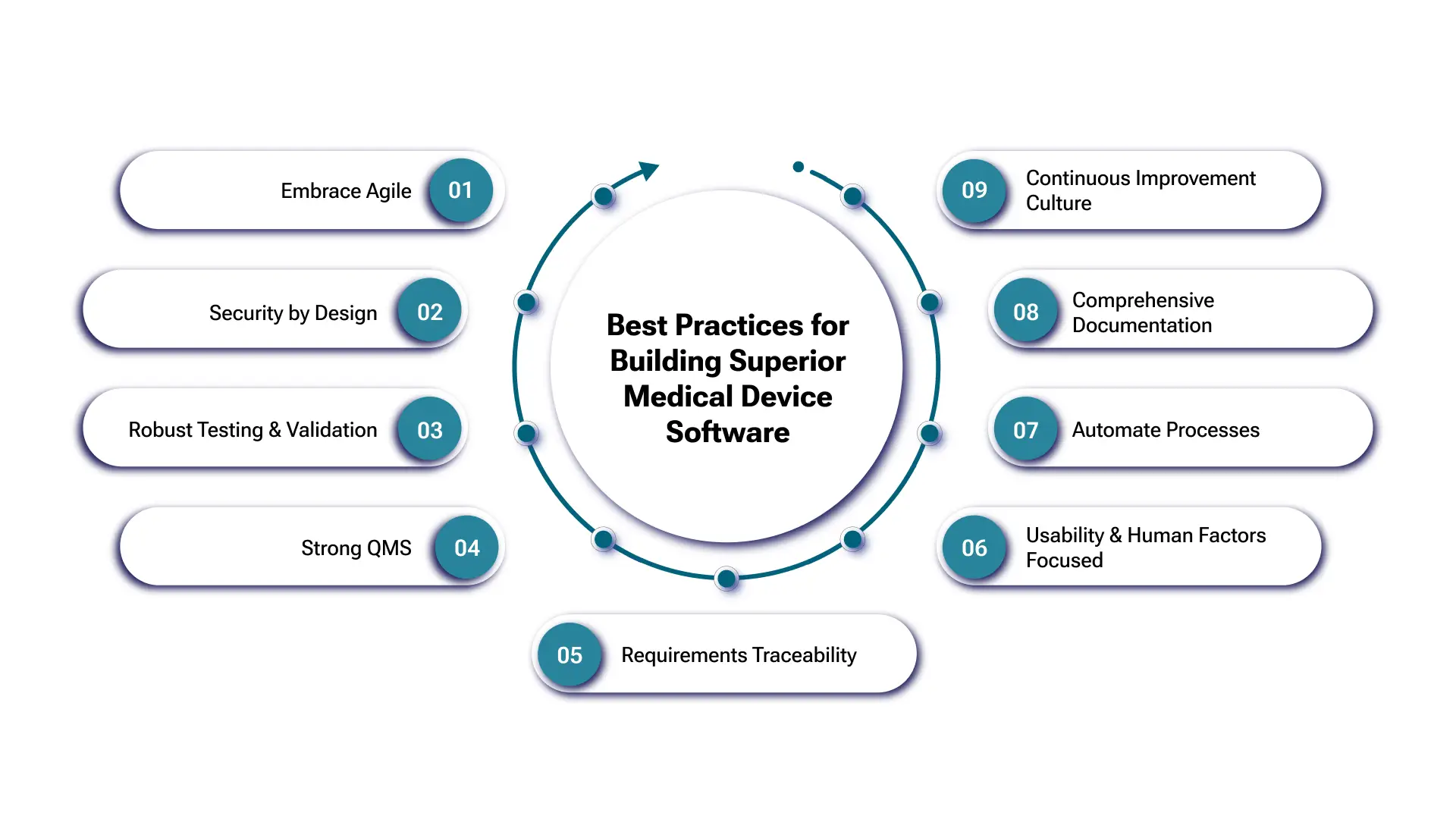
Embrace Agile
Use Agile methodologies (Scrum, Kanban) for iterative development, flexibility, and faster cycles. Agile enhances collaboration and adapts to evolving needs.
Security by Design
Integrate medical device cybersecurity from the start. Conduct early risk assessments and implement security controls at every layer. Regular security testing is vital.
Robust Testing & Validation
Thorough medical device software testing is non-negotiable. Employ multi-layered testing (unit, integration, system, usability, security). Validate software for intended clinical use.
Strong QMS
Establish a robust quality management system that is aligned with medical device software standards like ISO 13485. A QMS ensures consistency, traceability, and continuous improvement across the lifecycle.
Requirements Traceability
Maintain full traceability from requirements to code and testing. Essential for compliance, impact analysis, and ensuring complete requirement coverage. Utilize specialized requirements management tools (e.g., Jama Connect, Helix ALM) to manage traceability matrices effectively—Automate documentation generation where possible to reduce manual effort and ensure consistency.
Usability & Human Factors Focused
Design for clinicians and patients. Prioritize usability testing and human factors engineering for intuitive, efficient, and safe software in clinical settings.
Automate Processes
Automate testing, builds, and deployments. Automation boosts efficiency, reduces errors, and improves consistency in medical device software testing and development.
Comprehensive Documentation
Maintain clear documentation throughout development (requirements, design, testing, risk). Crucial for compliance, maintenance, and knowledge transfer.
Continuous Improvement Culture
Regularly refine processes and QMS based on feedback and evolving best practices. Use post-market data to drive iterative software enhancements.
Implementing these best practices significantly improves the quality, safety, and compliance of your medical device software development services, facilitating market success and positive patient impact.
Latest Advancements in Medical Device Software Development
Medical device software development is rapidly evolving. Staying updated on these trends is vital for startup owners and CXOs to remain competitive.
AI & ML Integration
AI/ML are core now, revolutionizing diagnostics, treatment, RPM, and personalized medicine. Advancements enable sophisticated applications like automated image analysis.
Cloud & SaaS
Cloud platforms are gaining traction in the medical device software sector, offering scalability, accessibility, and cost savings through SaaS models. Enables RPM and digital therapeutics innovation.
IoMT & Connected Devices
Medical device software development companies are seeing an explosion of IoMT, which generates vast amounts of data. Medical device software analyzes this for RPM, personalized insights, and proactive health management fueled by wireless and data analytics.
Digital Therapeutics (DTx)
DTx, as software as a medical device, emerges as evidence-based interventions delivered via apps for chronic conditions, mental health, and more – offering scalable, cost-effective treatments.
Personalized Medicine Software
The software analyzes individual data (genomics, history) for tailored treatments, predicting responses, optimizing dosages, and guiding precision medicine.
Cybersecurity & Data Privacy Focus
Cybersecurity and data privacy are paramount. Advancements in medical device software development services, security tech, encryption, and privacy-preserving methods are crucial amidst rising cyber threats and regulations.
Development Tool & Automation Advancements
Improved tools, automation, and Model-Based Design streamline software development for medical devices, boosting efficiency, quality, and speed to market with automated testing and code generation.
By tracking these advancements and integrating relevant tech, startup owners and CXOs can drive success in the dynamic medical device software landscape.
Transform Your Medical Device Software Vision with VLink
Are you struggling with complex medical device software development? VLink, a leader in healthcare software development services, helps startup owners and CXOs like you bring software as a medical device (SaMD) to life faster and with less risk.
We offer custom software development services with deep expertise in medical device software standards, medical device cybersecurity, and the software risk management process. Whether it's DICOM medical imaging software or remote patient monitoring software, VLink provides the regulatory knowledge and technical skills you need.
Partner with VLink to:
- Accelerate innovation & reduce risk.
- Navigate complex regulations confidently.
- Build robust cybersecurity from day one.
- Focus on your vision; we handle the tech.
- Get scalable solutions for startups and enterprises.
Conclusion
Medical device software development is at the exciting crossroads of tech innovation and healthcare evolution. From AI diagnostics to remote patient monitoring and digital therapeutics, software as a medical device (SaMD) is poised to revolutionize patient care. This guide has highlighted the intricate journey, demanding careful planning, regulatory compliance, and unwavering safety commitment.
For startup owners and CXOs in this dynamic field, understanding these nuances and adopting best practices is key. While challenges like regulations and cybersecurity are real, the rewards – impactful solutions, improved outcomes, and market growth – are immense. As healthcare digitizes, the role of medical device software will only be amplified. Embrace innovation, prioritize quality, and navigate regulations strategically to succeed and contribute to a healthier future globally.
Ready to bring your medical device software vision to life? Contact VLink today to explore how our expert services can help you navigate this exciting landscape.










 Shivisha Patel
Shivisha Patel

















